Weiterführende Informationen
Wissenschaftliche Einrichtungen
Mikrobiologie
Inhalte
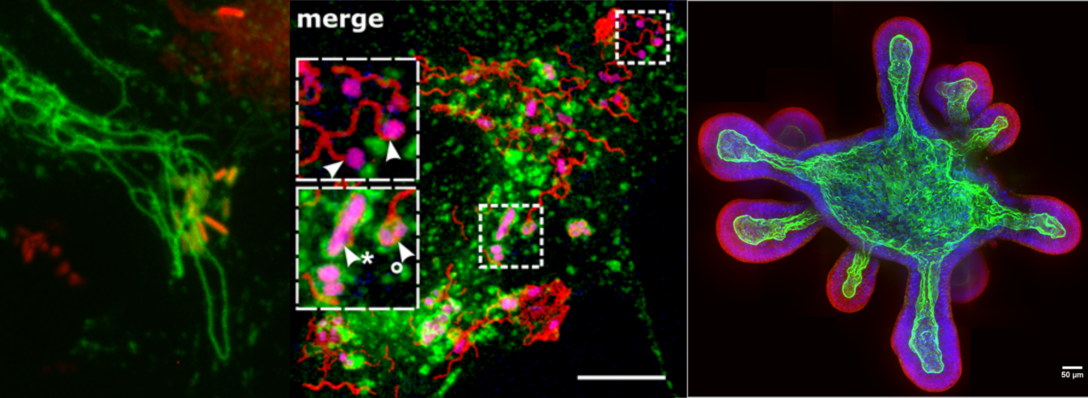
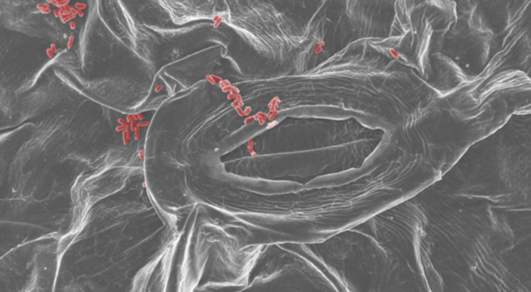
Die Abteilung Mikrobiologie untersucht molekulare Funktionen bakterieller Zellen. Dabei interessieren uns Fragestellungen der Pathogenität von Bakterien wie auch grundlegende Zellfunktionen wie der Aufbau komplexer molekularer Maschinen.
Topinformationen
Aktuelle Informationen
Aktuell werden alle Klausuren in der Mikrobiologie in unseren E-Prüf-Räumen in Gebäude 94 in Vips in StudIP geschrieben.
Weitere Informationen zu unseren Forschungsschwerpunkten
Forschungszentrum CellNanOs - Elektronenmikroskopie
Forschungskooperation Israel